Research
1. Polymers at Interfaces
2. Binding of Molecules to Functionalized Monolayers
3. Nanoparticle - Liquid Crystalline Materials
1. Polymers at Interfaces
Over the last decade, solid state NMR spectroscopy has proven to be a powerful method for revealing the chain level behavior responsible for the properties of bulk solid polymers. We are one of the few groups to apply these techniques to to understand interesting phenomema observed for polymers at interfaces.
We ask questions such as:
- How are the adhesive properties of certain copolymers related to the surface chain configuration and mobility?
- Are the polymers at air/solid interface in a rubbery or glassy state?
- And what happens when this surface has a very high curvature as in a nanoparticle substrate?
Polymer encapsulated nanoparticles:
13C and 2H solids NMR spectroscopies were combined with UV-vis spectroscopy, electrophoresis and transmission electron microscopy to understand the adsorption of charged polymers on gold nanoparticles stabilized by labile ligands (DMAP). Does the polyelectrolyte displace or associate with DMAP? What is the effect of the high surface curvature?
For the answers, see Langmuir 2008 vol. 24, 2532.
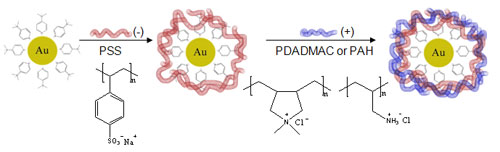
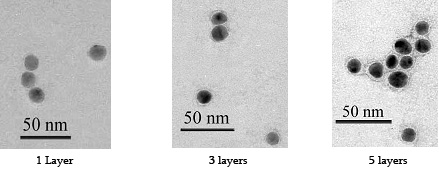
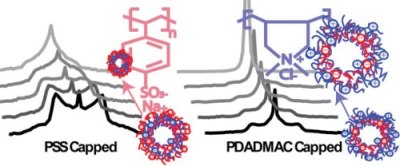
On going work concerns the deposition of multilayers to see how small we can go. Below are images of 13 nm gold nanoparticles encapsulated by multilayers:
Multilayer polymer capsules:
Polymer microcapsules can be formed by the sequential adsorption of charged and hydrogen bonding polymers on colloidal substrates followed by the dissolution of the solid cores. In addition to the obvious applications in biotechnology and separations science, these new materials display interesting responses to physical stimuli such as ionic strength and temperature.
We examined the chain dynamics underlying this behavior for polyelectrolyte multilayers by correlating wideline 2H NMR with sensitive calorimetric measurements.
See Macromolecules, 2009, vol. 42 , p. 247
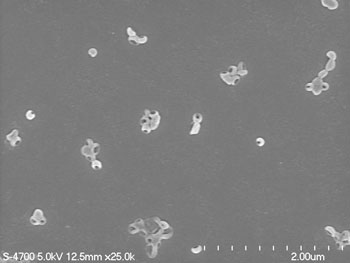
Ongoing work concerns hydrogen bonding polymer supported multilayers and capsules using both strong and weak hydrogen bond acceptor-donor polymer pairs. Below is a SEM image of PVP-PMAA submicron capsules which collapse upon drying.
2. Binding of Molecules to Functionalized Monolayers
Self-assembled monolayers (SAMs) are widely used to produce well defined arrays of surface functionalities. Our group has extensively characterized the conformational and dynamic properties of alkane based monolayers. A recent study showed that solids NMR can detect and quantify interparticle interactions of SAM modified nanoparticles.
For details see Langmuir, 2008, vol. 24, p. 2465.

Biotechnology and sensor applications of SAMs often require selective yet reversible binding of small molecules, polymers or colloidal particles through noncovalent interactions. The design of such systems will greatly benefit from molecular level characterization since the association of surface bound functionalities with other molecules may differ from the bulk state analogues. High resolution solids NMR techniques, based on “through space” dipolar interactions, can directly detect the specific noncovalent interactions.

See our earlier work: J. Am. Chem. Soc., 2003, vol. 125, p. 4174
3. Nanoparticle - Liquid Crystalline Materials
Liquid crystal (LC)-nanoparticle blends present fascinating fundamental and applications problems. The suspended nanoparticles introduce distortions and defects in the ordered LC hosts, inducing new inter-particle forces that are completely unique to organized fluid media. Our work focused on creating stable, regular and long-range assembly of individual nanoparticles using a liquid crystal (LC) as an organic template. We studied the organized structure by optical microscopy and its static and dynamic properties by solid-state NMR techniques. Our strategy was five folds:
(1) Tuning the miscibility of gold nanoparticles in liquid crystal medium. We developed a chemical method to produce compositionally tailored capping layers at the surface of gold nanoparticles (AuNP) to create a stable dispersion of individual particles in an isotropic LC (Fig. 1). J. Mater. Chem., 2011, 21, 9043
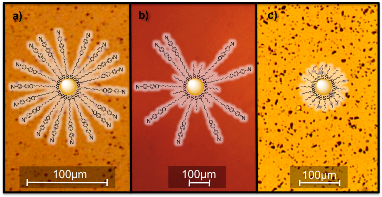
Figure 1 Polarized Optical Microscopy (POM) images of 4-5 diameter AuNP in isotropic 4-n-pentyl-4’-cyanobiphenyl (5CB) LC. (b) We achieved unprecedented AuNP miscibility up to 25wt% Au by capping the particles with a mixed monolayer made of mesogenic 4’-(n-dodecyloxy)biphenyl-4-carbonitriles (CBO(CH2)12SH) and hexanethiol CH3(CH2)5SH ligands. Monolayer-capped AuNP with (a) CBO(CH2)12SH and (c) CH3(CH2)5SH ligands have low miscibility and form aggregates.
(2) Reversible long-range assembly of nanoparticles in liquid crystal medium. We assembled the functionalized AuNPs using the long-range order of LC through a phase separation process at the isotropic-to-nematic (Fig. 2, click here for the video) and nematic-to-smectic (Fig. 3, click here for video) phase transitions of LC. Soft Matter, 2012, 8, 173
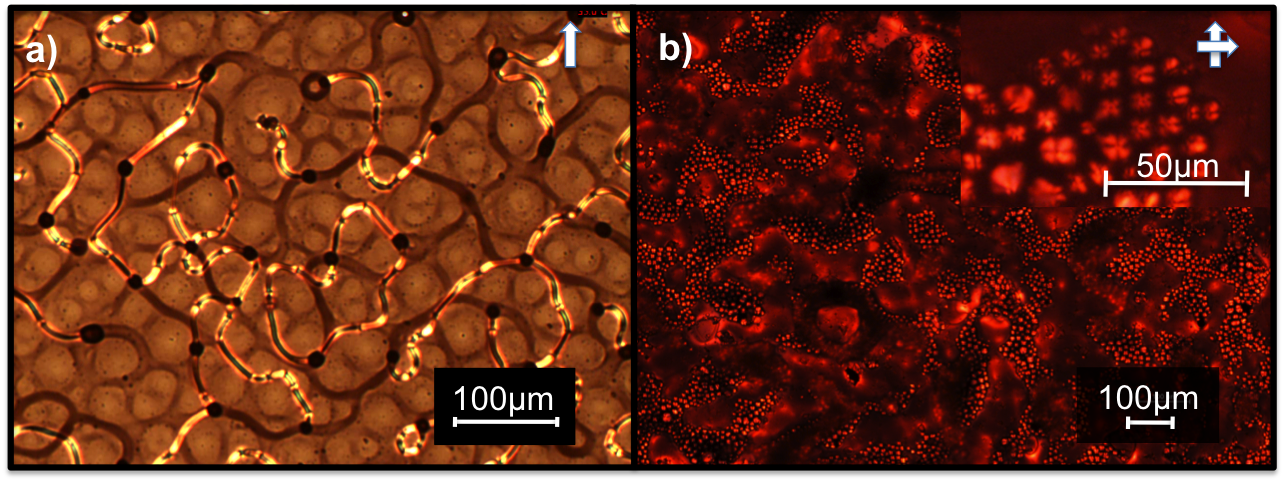
Figure 2 POM images of AuNP networks in nematic 5CB LC. We controlled the network topology and LC director field orientation by varying the film thickness between (a) 5 μm and (b) 70 μm, cooling rate, surface alignment, AuNP concentration and ligand shell composition.
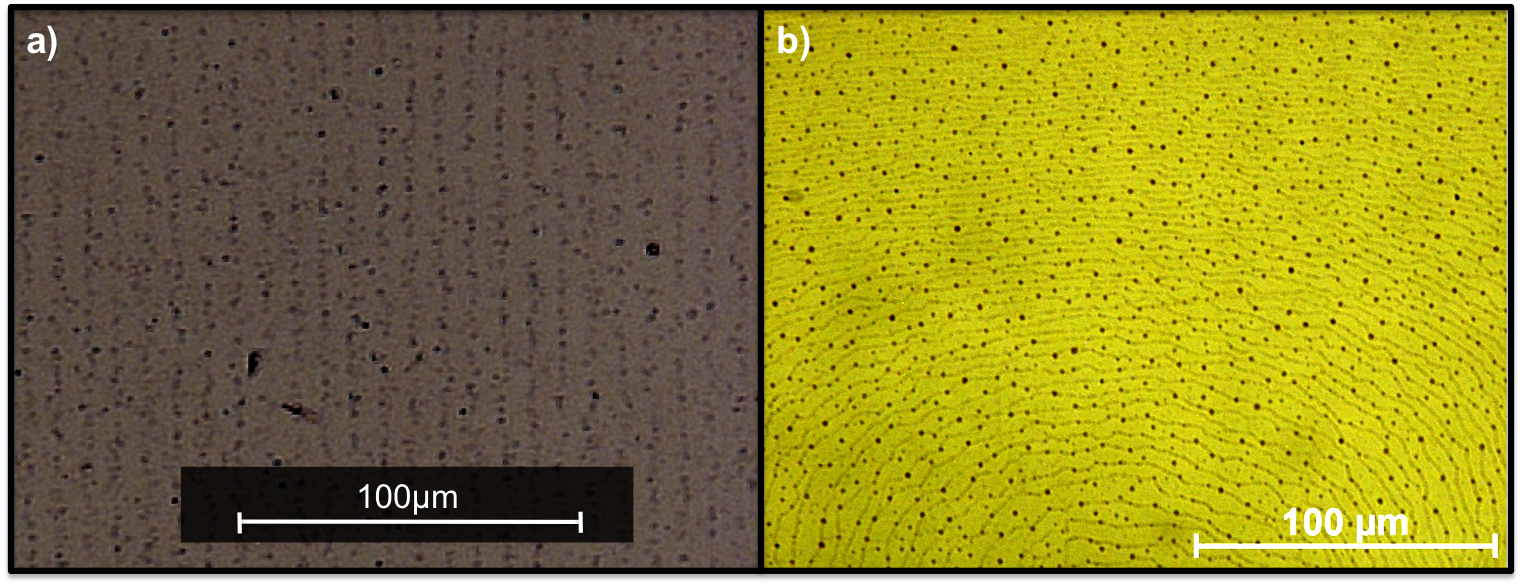
Figure 3 POM images of AuNP arrays in smectic 8CB LC. We controlled the geometry and spacing of the mm2 size arrays by using (a) a wedge cell and (b) a cavity cell forming a meniscus with homeotropic LC alignment.
(3) Solid-state NMR characterization of the nanoparticle-liquid crystal composites. We studied the static and dynamic properties of the gold nanoparticle organic capping layer by 1H, 2H and 13C NMR. As for the composites, wideline 2H NMR with isotopically labeled AuNP ligands and 5CB was used to selectively probe the mobility and order of the ligands and host LC as a function of temperature and NP concentration (Fig. 4). 2H NMR was also used to study the effect of a hydrophobic and hydrophilic silica network on the orientational order of a Schiff-base-type of LC (Fig. 5). J. Phys. Chem. B, 2008, 112, 3322
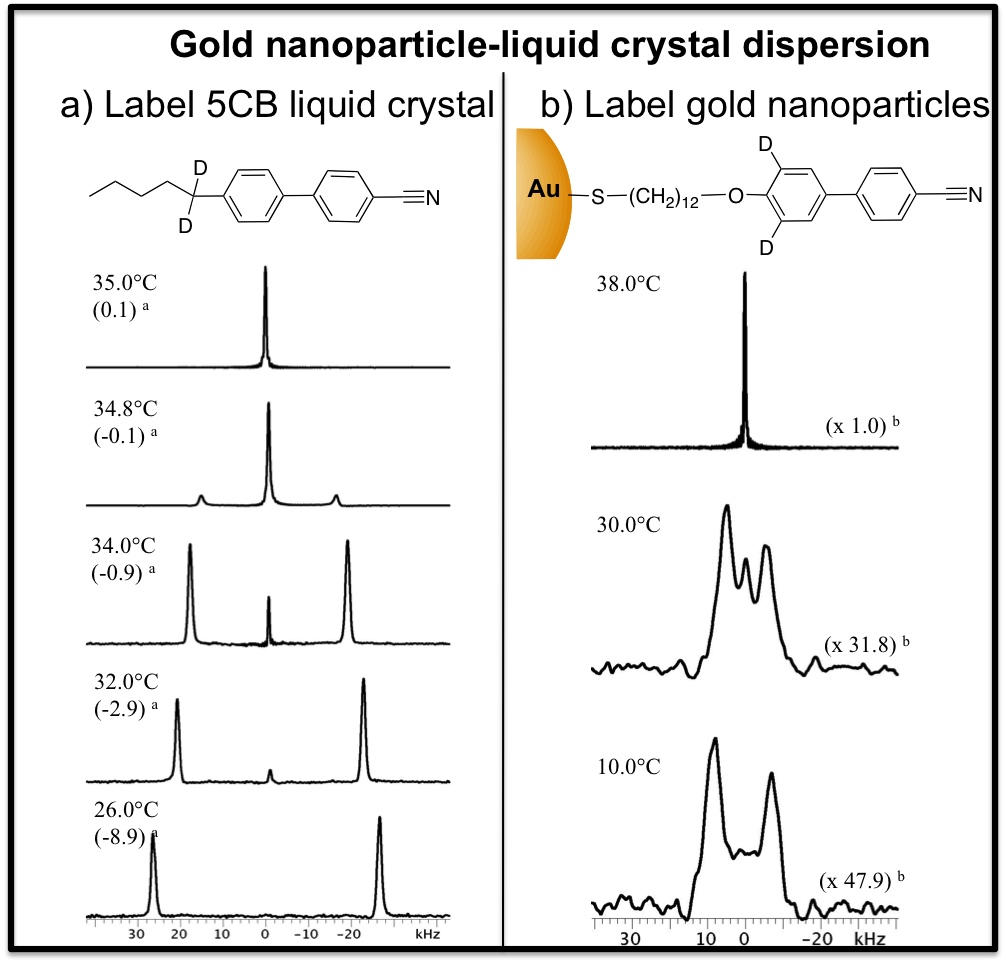
Figure 4 2H solid-state NMR of 13wt% AuNP dispersion in 5CB LC. (a) We detected an isotropic (NMR singlet) and nematic (NMR doublet) 5CB component below TN-I and (b) the alignment of the mesogenic ligand (NMR doublet) at the surface of the AuNP. This is in agreement with the distortion of the AuNP into a tactoidal shape when dispersed in LC where he LC ligands at the “poles” of the NP are extended while those at the equator have a greater density of bent (gauche) conformers.
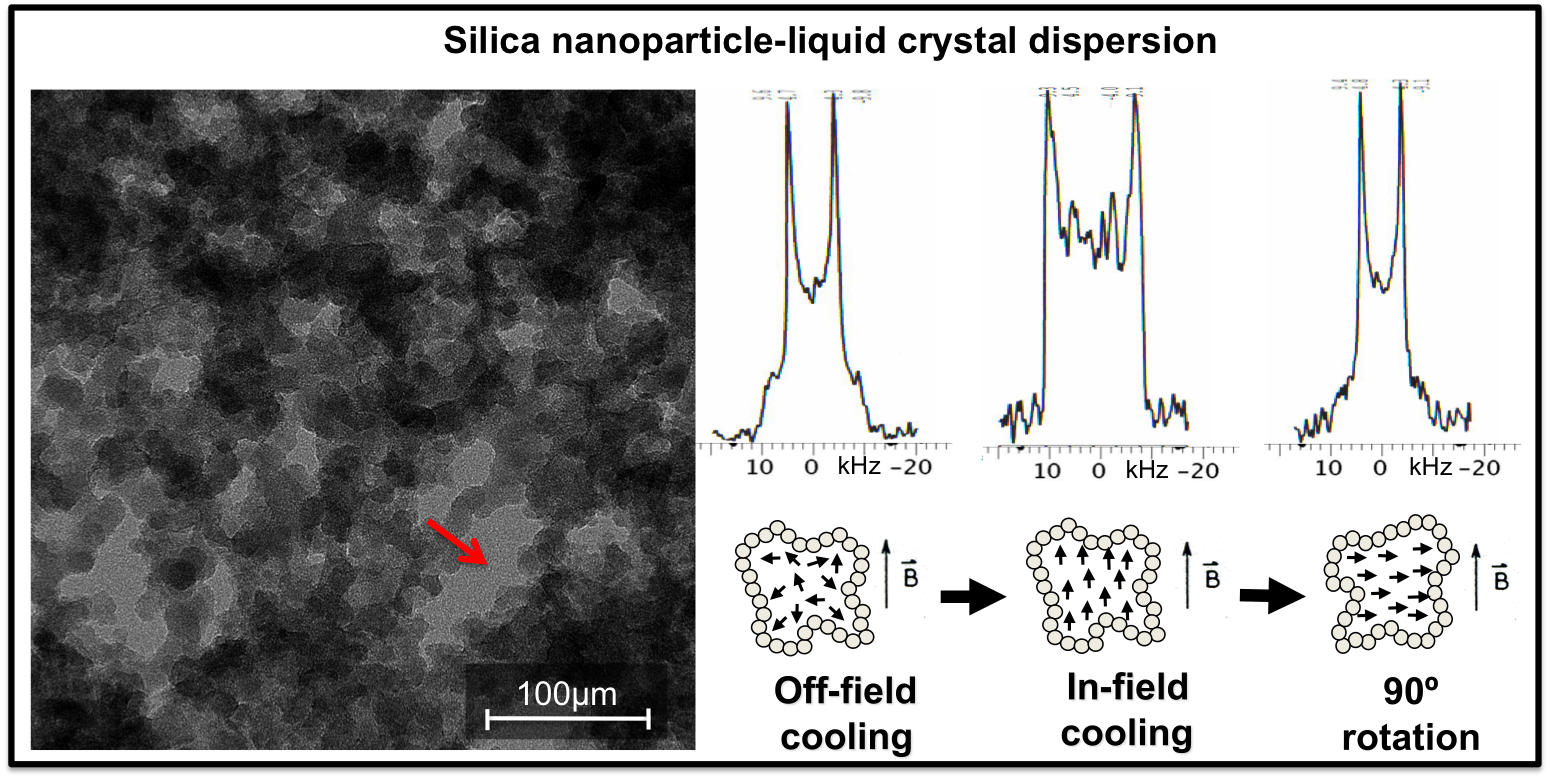
Figure 5 Cryo-Electron Microscopy image (left) and 2H solid-state NMR (right) of aerosil dispersion in p-ethoxy(benzylidene)-p-n-butylaniline (2O.4), EBBA, a liquid crystal with a low dipole moment. Silica aerosils in LC consist of irregularly branched strings of spherical, nanoparticles of amorphous silica linked together by -Si-O-Si- bonds forming an aerogel-like network of void spaces (see red arrow) that are hosting the LC. After in-field cooling, the LC director orientation is locked in the cavities and follows the sample orientation in the field resulting in a distorted 2H NMR Pake pattern (see cartoons of cavities: circle = silica nanoparticles, black arrows = orientation of nematic domains). We discovered that the memory effect, the persistence of this aligned state with out a field, is stronger for hydrophilic silica than for hydrophobic one.
(4) Experiments and theory of gold nanoparticle-nematic liquid crystal composites. In order to better understand the long-range assembly of gold nanoparticles in liquid crystal, we combined our experimental data with theoretical ones and study the effect of nanoparticle shape on the organized structure.In collaboration with the McGill University Modeling Research Group lead by Prof. Alejandro Rey (link), we combined experimental and theoretical data to study the effect of NP monolayer composition and concentration in LC medium on their assembly in cellular network (Fig. 6). Soft Matter, 2012, DOI:10.1039/C2SM07091J
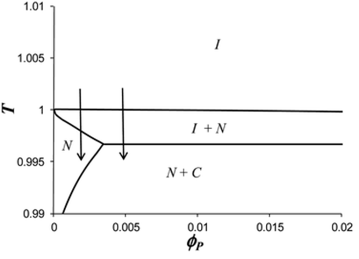
Figure 6 Theoretical phase diagram (temperature T in function of concentration ϕ p in 5CB) for mix monolayer gold nanoparticles with 70% surface coverage of mesogenic ligand. Mean field theory predict four phases depending on temperature: isotropic (I), nematic (N), crystalline (C) and nematic-crystal (NC). We found that the structure of the composite depends on the phase equilibrium behavior.
(5) Tuning the miscibility of gold nanorods in liquid crystal medium. We synthesized CTAB (cetyltrimethylammonium bromide) stabilized gold nanorods (AuNR) and used a two phases thiol-for-CTAB exchange reaction to totally remove the cytotoxic CTAB surfactant and tuned the mix monolayer at the gold surface (Fig. 7a). The solubility and assembly of the AuNR were tested in 5CB and E7 liquid crystals (Fig. 7b).
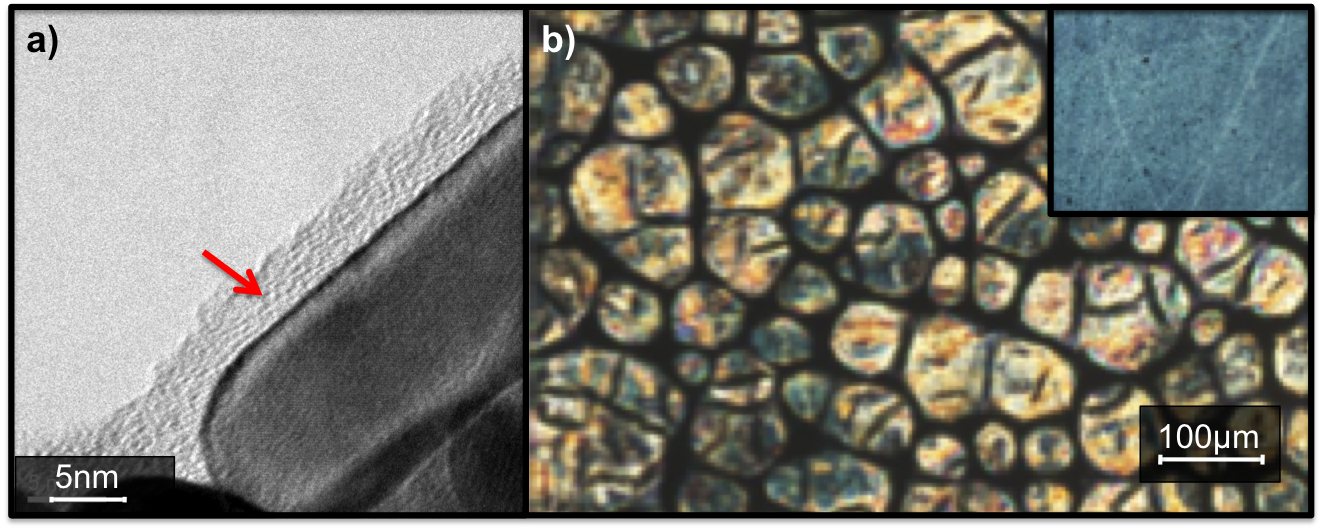
Figure 7 (a) High resolution TEM of gold nanorods done in collaboration with the York University JEOL Nanocenter lead by Prof. Pratibha Gai (link) (red arrow = organic mix monolayer coating the rods). (b) Polarized Optical Microscopy of gold nanorods-E7 liquid crystal dispersion forming a cellular network at TN-I (inset = isotropic dispersion).